Content

Abstract
Electricity is no more a luxury but it has become a necessary in today’s life. An increase in share of global energy needs is expected to be met by renewable in the years ahead. Renewable sources have an enormous potential to meet the growing energy requirements of the increasing population of the developing world.
Fuel cells is one of them, provide a range of critical benefits that no other single power generating technology can match.
This technical article describes the main characteristics of fuel cell and in that mainly Solid Oxide Fuel Cell (SOFC).
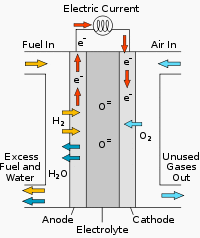
High temperature solid oxide fuel cells (SOFCs) offer a clean, pollution-free technology to electrochemically generate electricity at high efficiencies.
High temperature solid oxide fuel cell (SOFC) technology is a promising power generation option that features high electrical efficiency and low emissions of environmentally polluting gases such as CO2, NOx and SOx. SOFCs are suitable for stationary applications as well as for auxiliary power units (APUs) used in vehicles to power electronics.
Much development has focused on solid oxide fuel cells (SOFC) because it is able to convert a wide variety of fuels and with such high efficiency.
Introduction
Engineers and environmentalists have long dreamed of being able to obtain the benefits of clean electric power without pollution-producing engines or heavy batteries. Solar panels and wind farms are familiar images of alternative energy technologies. While they are effective sources of electrical energy, there are problems with the stability of their energy source as, for example, on a cloudy or windless day.
Their applications are somewhat limited due to lack of portability; a windmill is not much help to the power plant of a diesel truck, a solar panel cannot provide power at night, etc.
In 1962 a revolution in energy research occurred. Scientists at Westinghouse Electric Corporation (now Siemens Westinghouse) demonstrated for the first time the feasibility of extracting electricity from a device they called a “solid electrolyte fuel cell”. Since then there has been an intense research and development effort to develop the alternative energy technology known as fuel cells.
Now, as energy issues are at the forefront of current events, fuel cell technology is ripening and on the verge of being ready for large scale commercial implementation.
Fuel Cell
A fuel cell is an electrochemical device that converts the chemical energy in fuels (such as hydrogen, methane, butane or even gasoline and diesel) into electrical energy by exploiting the natural tendency of oxygen and hydrogen to react. By controlling the means by which such a reaction occurs and directing the reaction through a device, it is possible to harvest the energy given off by the reaction.
Highly efficient hydrogen fuel cells are wanted due to the high price the existing ones have so far. So, being efficient means less money spent on them, and more market share for hydrogen. SOFCs (solid oxide fuel cell) are a type of hydrogen fuel cell that use solid (not liquid) electrolyte to do their job, while being much more efficient.
SOFC technology dominates competing fuel cell technologies because of the ability of SOFCs to use currently available fossil fuels, thus reducing operating costs. Other fuel cell technologies (e.g. molten carbonate, polymer electrolyte, phosphoric acid and alkali) require hydrogen as their fuel.
Working Principle of SOFC
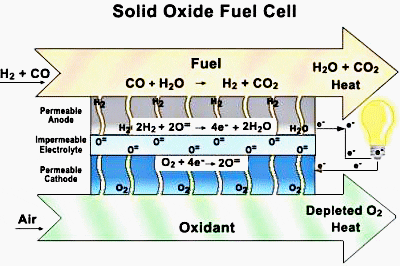
Figure 1 above shows schematically how a solid oxide fuel cell works. The cell is constructed with two porous electrodes which sandwich an electrolyte. Air flows along the cathode (which is therefore also called the “air electrode”). When an oxygen molecule contacts the cathode/electrolyte interface, it catalytically acquires four electrons from the cathode and splits into two oxygen ions.
The oxygen ions diffuse into the electrolyte material and migrate to the other side of the cell where they encounter the anode (also called the “fuel electrode“).
The oxygen ions encounter the fuel at the anode/electrolyte interface and react catalytically, giving off water, carbon dioxide, heat, and – most importantly for a cycle two electrons. The electrons transport through the anode to the external circuit and back to the cathode, providing a source of useful electrical energy in an external circuit.
[]
<h3″>Materials Selection and Processing
Although the operating concept of SOFCs is rather simple, the selection of materials for the individual components presents enormous challenges. Each material must have the electrical properties required to perform its function in the cell. There must be enough chemical and structural stability to endure fabrication and operation at high temperatures.
Reactivity and interdiffusion between the components must be as low as possible. The thermal expansion coefficients of the components must be as close to one another as possible in order to minimize thermal stresses which could lead to cracking and mechanical failure. The air side of the cell must operate in an oxidizing atmosphere and the fuel side must operate in a reducing atmosphere. The temperature and atmosphere requirements drive the materials selection for all the other components.
In order for SOFCs to reach their commercial potential, the materials and processing must also be cost-effective. The first successful SOFC used platinum as both the cathode and anode, but fortunately less expensive alternatives are available today.
Fuel cells are simple devices, containing no moving parts and only four functional component elements: cathode, electrolyte, anode and interconnection.
Cathode
The cathode must meet all the above requirements and be porous in order to allow oxygen molecules to reach the electrode/electrolyte interface. In some designs (e.g. tubular) the cathode contributes over 90% of the cell’s weight and therefore provides structural support for the cell.
Materials used for Catode
Today the most commonly used cathode material is lanthanum manganite (LaMnO3), a p-type perovskite. Typically, it is doped with rare earth elements (e.g. Sr, Ce, Pr) to enhance its conductivity. Most often it is doped with strontium and referred to as LSM (La1-xSrxMnO3).
The conductivity of these perovskites is all electronic (no ionic conductivity), a desirable feature since the electrons from the open circuit flow back through the cell via the cathode to reduce the oxygen molecules, forcing the oxygen ions through the electrolyte. In addition to being compatible with YSZ electrolytes, it has the further advantage of having adequate functionality at intermediate fuel cell temperatures (about 700 C), allowing it to be used with alternative electrolyte compositions.
Any reduction in operating temperature reduces operating costs and expands the materials selection, creating an opportunity for additional cost savings.
Fabrication of LSM depends on cell design. For example, the tubular cell is constructed by extruding a cathode tube and building the rest of the cell around it, where several planar cell designs are being investigated, the cathode is designed as the bottom supporting layer, and fabricated with tape casting techniques using nanoscale particles. In both cases, the challenge is to sinter the cathode adequately, often by co-sintering with the other components, while maintaining sufficient interconnected porosity.
Electrolyte
Once the molecular oxygen has been converted to oxygen ions it must migrate through the electrolyte to the fuel side of the cell. In order for such migration to occur, the electrolyte must possess a high ionic conductivity and no electrical conductivity. It must be fully dense to prevent short circuiting of reacting gases through it and it should also be as thin as possible to minimize resistive losses in the cell.
There are several candidate materials: YSZ, doped cerium oxide, and doped bismuth oxide. Of these, the first two are the most promising. Bismuth oxide-based materials have a high oxygen ion conductivity and lower operating temperature (less than 800 C), but do not offer enough crystalline stability at high temperature to be broadly useful.
YSZ has emerged as the most suitable electrolyte material. Yttria serves the dual purpose of stabilizing zirconia into the cubic structure at high temperatures and also providing oxygen vacancies at the rate of one vacancy per mole of dopant. A typical dopant level is 10mol% yttria.
If the conductivity for oxygen ions in SOFC can remain high even at lower temperature, material choice for SOFC will broaden and many existing problems can potentially be solved. Certain processing technique such as thin film deposition can help solve this problem with existing material by:
- Reducing the traveling distance of oxygen ions and electrolyte resistance as resistance is inversely proportional to conductor length;
- Producing grain structures that are less resistive such as columnar grain structure;
- Controlling the micro-structural nano-crystalline fine grains to achieve “fine-tuning” of electrical properties;
- Building composite with large interfacial areas as interfaces have shown to have extraordinary electrical properties.
Cerium oxide has also been considered as a possible electrolyte. Its advantage is that it has high ionic conductivity in air but can operate effectively at much lower temperatures(under 700 C); this temperature range significantly broadens the choice of materials for the other components, which can be made of much less expensive and more readily available materials.
Anode
The anode (the fuel electrode) must meet most of the same requirements as the cathode for electrical conductivity, thermal expansion compatibility and porosity, and must function in a reducing atmosphere. The reducing conditions combined with electrical conductivity requirements make metals attractive candidate materials.
Most development has focused on nickel owing to its abundance and affordability. The most common material used is a cermet made up of nickel mixed with the ceramic material that is used for the electrolyte in that particular cell, typically YSZ (yttria stabilized zirconia), this YSZ part helps stop the grain growth of Nickel Ni.
The YSZ provides structural support for separated Ni particles, preventing them from sintering together while matching the thermal expansions. Adhesion of the anode to the electrolyte is also improved.
Anodes are applied to the fuel cell through powder technology processes. Either slurry of Ni is applied over the cell and then YSZ is deposited by electrochemical vapor deposition, or Ni-YSZ slurry is applied and sintered. More recently NiO-YSZ slurries have been used, the NiO being reduced to particulate Ni in the firing process.
Although Ni-YSZ is currently the anode material of choice and the freeze-drying process solves most of the associated problems, nickel still has a disadvantage: it catalyzes the formation of graphite from hydrocarbons. The deposition of graphite residues on the interior surfaces of the anode reduces its usefulness by destroying one of the main advantages of SOFCs, namely their ability to use unreformed fuel sources.
Cu-cerium oxide anodes are being studied as a possible alternative. Copper is an excellent electrical conductor but a poor catalyst of hydrocarbons; cerium oxide is used as the matrix in part because of its high activity of hydrocarbon oxidation. A composite of the two thus has the advantage of being compatible with cerium oxide electrolyte fuel cells. Initial results using a wide range of hydrocarbon fuels are promising.
Interconnection
The interconnection serves as the electrical contact to the cathode while protecting it from the reducing atmosphere of the anode.
The requirements of the interconnection are the most severe of all cell components and include the following:
- 100% electrical conductivity,
- No porosity (to avoid mixing of fuel and oxygen),
- Thermal expansion close to that of the air electrode and the electrolyte. compatibility, and
- Inertness with respect to the other fuel cell components.
- It will be exposed simultaneously to the reducing environment of the anode and the oxidizing atmosphere of the cathode.
To satisfy these requirements, doped lanthanum chromite is used as the interconnection material. Ca-doped yttrium chromite is also being considered because it has better thermal expansion compatibility, especially in reducing atmospheres. At operating temperatures in the 900-1000 C range interconnects made of such nickel base alloys as Inconel 600 is possible.
At or below 800 C, ferritic steels can be used. At even lower temperatures (below 700 C), it becomes possible to use stainless steels, which are comparatively inexpensive and readily available.
Types of SOFC
Two possible design configurations for SOFCs have emerged:
- Planar design (Figure 2)
- Tubular design (Figure 3)
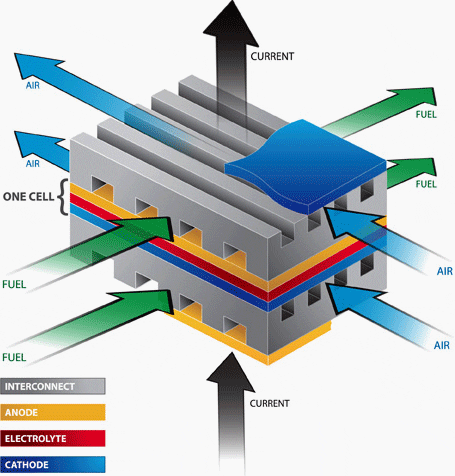
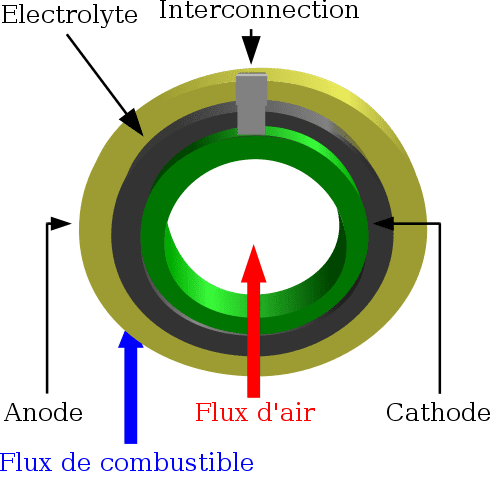
In the planar design, the components are assembled in flat stacks, with air and fuel flowing through channels built into the cathode and anode. In the tubular design, components are the cell constructed in layers around a tubular cathode; air flows through the inside of the tube and fuel flows around the exterior.
Merits
- High efficiency
- Fuel adaptability
- SOFCs are attractive as energy sources because they are clean, reliable, and almost entirely nonpolluting.
- If the hydrogen used comes from the electrolysis of water, then using fuel cells eliminates greenhouse gases.
- Because there are no moving parts and the cells are therefore vibration-free, the noise pollution associated with power generation is also eliminated.
- By using SOFC in CHP to reduce the emissions resulting in Zero Emission Power Generation.
Demerits
- The largest disadvantage is the high operating temperature which results in longer start-up times and mechanical and chemical compatibility issues.
- Fuelling fuel cells is still a problem since the production, transportation, distribution and storage of hydrogen is difficult
Applications
SOFCs are targeted for use in three energy applications: stationary energy sources, transportation, and military applications.
Stationary energy sources
Stationary installations would be the primary or auxiliary power sources for such facilities as homes, office buildings, industrial sites, ports, and military installations. They are well suited for mini-power-grid applications at places like universities and military bases.
SOFCs can be positioned on-site, even in remote areas; on-site location makes it possible to match power generation to the electrical demands of the site. Stationary SOFC power generation is no longer just a hope for the future.
Transportation
In the transportation sector, SOFCs are likely to find applications in both trucks and automobiles.
In diesel trucks, they will probably be used as auxiliary power units to run electrical systems like air conditioning and on-board electronics thereby leading to a savings in diesel fuel expenditures and a significant reduction in both diesel exhaust and truck noise.
Military applications
Finally, SOFCs are of high interest to the military because they can be established on-site in remote locations, are quiet, and non-polluting. Moreover, the use of fuel cells could significantly reduce deployment costs: 70% by weight of the material that the military moves is nothing but fuel.
Stationary fuel cells for military applications can provide back up or standby power for special operations and activities and can provide power in remote areas.
SOFC-GT
An SOFC-GT system is one which comprises a solid oxide fuel cell combined with a gas turbine. Further combination of the SOFC-GT in a combined heat and power plant also has the potential to yield even higher thermal efficiencies in some cases. In these plant SOFC is using as a replacement to combustor near gas turbine.
Within the SOFC module the desulfurized fuel is utilized electrochemically and oxidized below the temperature for NOx generation. Therefore NOx and SOx emissions for the SOFC power generation system are near negligible. The byproducts of the power generation from hydrocarbon fuels that are released into the environment are CO2 and water vapor.
The development of methods to capture and sequester the CO2, resulting in a Zero Emission power generation system.
Conclusions
Forty years have passed since the first successful demonstration of a solid oxide fuel cell. Through ingenuity, materials science, extensive research, and commitment to developing alternative energy sources, that seed of an idea has germinated and is about to bloom into a viable, robust energy alternative. Materials development will certainly continue to make SOFCs increasingly affordable, efficient, and reliable.
The rapid increasing in technology definitely brings a change in the usage of this SOFC and also in the power generation sector. Ultimately helps in bringing Zero Emission Power Generation.
References:
• http://en.wikipedia.org/wiki/Solid_oxide_fuel_cell
• http://www.csa.com/discoveryguides/fuecell/
• http://www.pg.siemens.com/en/fuelcells
• http://www.seca.doe.gov/overview.html









Good Mr Vinod Reddy . . SOFC is the future technology and Time is the Factor ..Recently I attended a USER MEET at ARSI,Hyderabad and had very interesting discussion and updates.. If possible /interetced reach me me in 9392148281, and even can meet if you are in Hyderabad
We are going to venture in to SOFC
Pl share your contacts
Good luck..
K.Ramakrishnan
Vice President
Vijai Electricals ltd.
Hyderabad
At what point do we stop participating in creating false hope narratives? One photo of some shiny boxes and a lot of old stuff about “how fuel cells work” even though they don’t… and you have created click-bait for the hopeful. There is a movement in science and engineering to help us reform and be brutally honest about which technologies have potential and which don’t. Please everybody evolve and become Transition Engineers.
Thanks for your valuable suggestions Susan, You have to know one thing, this was written by me when i was doing undergraudate degree. Yes, technology will boom every day, every sec. If you have any thoughts, you can write an article continuing this so that our Engineers will have clear idea. See, the point idea may looks like old stuff, when it comes to reality, it requires a lot of effort and understanding.
Thanks for all !!
Hi, one thing that everyone overlooks and that is…..if the SOFC system has been on the boil for 40 years, why are we not seeing a usable device for every day electricity production……..Blue Gen is supposed to be the answer but is not even on the table for the Plebs.
Taken to the extreme….we are very comfortable with the petrol engine working at only 25% efficiency, and that has been for more than 100 years……steam engines in various forms produce the most power for electricity production and are the most user friendly of all devices.
When I read that 1,000 deg C is needed to make the SOFC more efficient, I have to realise that the plot has well and truly been lost……you might as well stick a pipe into all the volcanoes that are active and harness the free energy that goes to waste…..since time began.
That would be a technology that anyone can understand……….and you wouldn’t have to drill deep into the Earth’s mantle to reach the hot depths that geo thermal plants are doing.
Great ovreview Vinod, thank you.
Thanx for ur appreciation…:.!! I am thinking that the fuel cells also play am important role in ecofriendly generation of power..if the research scholars extend their work on fuel cells we will find better outcomes