 Sulphur hexafluoride gas (SF6) is employed as insulation in all parts of the installation, and in the circuit-breaker also for arc-quenching. SF6 is an electronegative gas, its dielectric strength at atmospheric pressure is approximately three times that of air.
Sulphur hexafluoride gas (SF6) is employed as insulation in all parts of the installation, and in the circuit-breaker also for arc-quenching. SF6 is an electronegative gas, its dielectric strength at atmospheric pressure is approximately three times that of air.
It is incombustible, non-toxic, odourless, chemically inert with arc-quenching properties 3 to 4 times better than air at the same pressure.
Commercially available SF6 is not dangerous, and so is not subject to the Hazardous Substances Order or Technical Regulations on Hazardous Substances (TRGS).
New SF6 gas must comply with IEC 60376 (VDE 0373 Part 1). Gas returned from SF6 installations and apparatus is dealt with in IEC 60480 (VDE 0373 Part 2). SF6 released into the atmosphere is considered a greenhouse gas.
With its contribution to the greenhouse effect below 0.1%, the proportion of SF6 is low compared to that of the better known greenhouse gases (carbon dioxide, methane, nitrous oxide etc.). To prevent any increase of SF6 in the atmosphere, its use should in future be confined to closed systems. Devices suitable for processing and storing SF6 gas are available for this purpose.
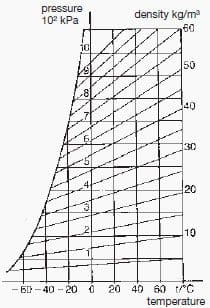
The gas pressure is monitored in the individually sealed gas compartments and in the circuit-breaker housing. The low gas losses (below 1 % per year) are taken into account with the first gas filling. Automatic make-up facilities are not necessary. The isolating gas pressure is generally 350 to 450 kPa at 20 °C. In some cases this can be up to 600 kPa. The quenching gas pressure is 600 to 700 kPa.
Outdoor apparatus exposed to arctic conditions contains a mixture of SF6 and N2, to prevent the gas from liquefying. The pressure-temperature relationship of pure SF6 gas is shown in Fig. 11-1.
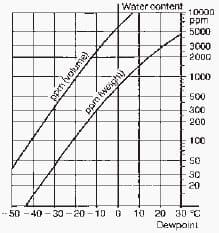
Arcing causes the decomposition of very small amounts of SF6 gas. The decomposition products react with water, therefore the gas’s moisture content, particularly in the circuit-breaker, is controlled by drying (molecular) filters.
Careful evacuation before first gas filling greatly reduces the initial moisture content. Fig. 11-2 illustrates the conversion of water vapour content into dewpoint.











@ Dean, yes SF6 is heavier then air, little over 5 times, however outside you must also consider atmosferical conditions will cause SF6 to mixt with the surrounding air. In closed rooms (eg. GIS buildings) the gas will drop to the bottom and if gastight, no aircirculation, it will stay there, in rest (eg. cable cellars) it is out most dangerous, in case of a leakage, it will push out ambient air making it impossible to breath. That is one of the reasons the pressure is monitored. FYI 1kg SF6 has a GWP of approx the equivalent of 22.300kg CO2
I’ve been wondering why, if SF-6 is quite a bit heavier than air, that it’s such an aggressive greenhouse gas. It shouldn’t be in the atmosphere as a blanket, but on the ground and sort of out of harms way. do you know why it’s considered a greenhouse problem if it should tend to stay on the ground?