
Introduction to Super Capacitors

Capacitors store electric charge. Because the charge is stored physically, with no chemical or phase changes taking place, the process is highly reversible and the discharge-charge cycle can be repeated over and over again, virtually without limit.
Electrochemical capacitors (ECs) variously referred to by manufacturers in promotional literature as Super capacitors also called ultra capacitors and Electric double layer capacitors (EDLC) are capacitors with capacitance values greater than any other capacitor type available today.
Capacitance values reaching up to 400 Farads in a single standard case size are available.
Super capacitors have the highest capacitive density available today with densities so high that these capacitors can be used to applications normally reserved for batteries. Super capacitors are not as volumetrically efficient and are more expensive than batteries but they do have other advantages over batteries making the preferred choice in applications requiring a large amount of energy storage to be stored and delivered in bursts repeatedly.
The super capacitors will supply power to the system when there are surges or energy bursts since super capacitors can be charged and discharged quickly while the batteries can supply the bulk energy since they can store and deliver larger amount energy over a longer slower period of time.
Construction of Super Capacitors
What makes’ super capacitors different from other capacitors types are the electrodes used in these capacitors. Super capacitors are based on a carbon (nano tube) technology. The carbon technology used in these capacitors creates a very large surface area with an extremely small separation distance.
Super capacitors do not have a traditional dielectric material like ceramic, polymer films or aluminum oxide to separate the electrodes, but instead have a physical barrier made from activated carbon that when an electrical charge is applied to the material a double electric field is generated which acts like a dielectric.
The thickness of the electric double layer is as thin as a molecule.
The surface area of the activated carbon layer is extremely large yielding several thousands of square meters per gram. This large surface area allows for the absorption of a large amount of ions.
The charging/discharging occurs in an ion absorption layer formed on the electrodes of activated carbon.
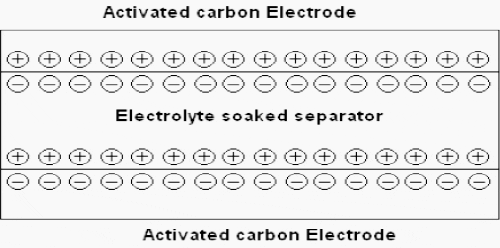
The activated carbon fiber electrodes are impregnated with an electrolyte where positive and negative charges are formed between the electrodes and the impregnant.
The electric double layer formed becomes an insulator until a large enough voltage is applied and current begins to flow. The magnitude of voltage where charges begin to flow is where the electrolyte begins to break down.
The double layers formed on the activated carbon surfaces can be illustrated as a series of parallel RC circuits.
As shown below the capacitor is made up of a series of RC circuits where R1, R2 …Rn are the internal resistances and C1, C2…, Cn are the electrostatic capacitances of the activated carbons.
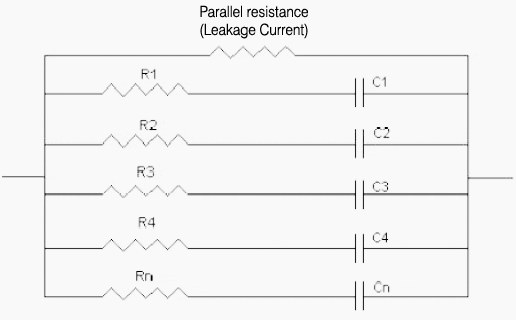
When voltage is applied current flows through each of the RC circuits. The amount of time required to charge the capacitor is dependent on the CxR values of each RC circuit.
Obviously the larger the CxR the longer it will take to charge the capacitor.
In= (V/Rn) exp (-t/ (Cn*Rn))
Super capacitor is a double layer capacitor; the energy is stored by charge transfer at the boundary between electrode and electrolyte. The amount of stored energy is function of the available electrode and electrolyte surface, the size of the ions, and the level of the electrolyte decomposition voltage.
Super capacitors are constituted of two electrodes, a separator and an electrolyte.
The two electrodes, made of activated carbon provide a high surface area part, defining so energy density of the component. On the electrodes, current collectors with a high conducting part assure the interface between the electrodes and the connections of the super capacitor. The two electrodes are separated by a membrane, which allows the mobility of charged ions and forbids no electronic contact.
The electrolyte supplies and conducts the ions from one electrode to the other.
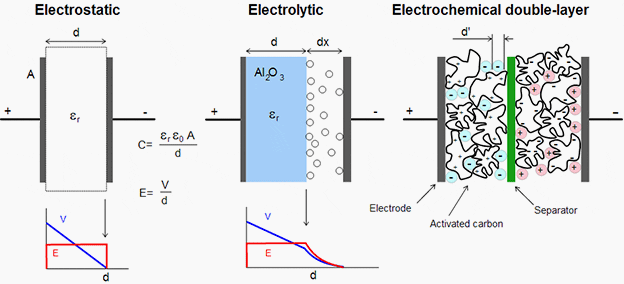
Usually super capacitors are divided into two types:
- Double-layer capacitors and
- Electrochemical capacitors
The former depends on the mechanism of double layers, which is result of the separation of charges at interface between the electrode surface of active carbon or carbon fiber and electrolytic solution. Its capacitance is proportional to the specific surface areas of electrode material.
The latter depends on fast faraday redox reaction.
Capacitance of them depends mainly on the utilization of active material of electrode.
The working voltage of electrochemical capacitor is usually lower than 3 V. Based on high working voltage of electrolytic capacitor, the hybrid super-capacitor combines the anode of electrolytic capacitor with the cathode of electrochemical capacitor, so it has the best features with the high specific capacitance and high energy density of electrochemical capacitor.
The capacitors can work at high voltage without connecting many cells in series.
The most important parameters of a super capacitor include the capacitance (C), ESR and EPR (which is also called leakage resistance).
Will be continued…











This is one site that lives up to its tittle. Thank you for the highly informative article.
thank you Vusa.
How much is it? Is some companies selling this supercapacitors?
thats very informative, i am really getting addicted to this site and like the fact that i have brought my friend to it.
thank you Mr. Evancoch