
It’s All About Colors…
Man has always been fascinated by light and has constantly striven to unravel its mysteries. History has produced various theories that today strike us as comical but were seriously propounded in their time.
For example, since no connection could be discerned between a flame and the object it rendered visible, it was at one time supposed that “visual rays” were projected by the eyes and reflected back by the object. Of course, if this theory were true, we would be able to see in the dark … :)
In 1675, by observing the innermost of the four large moons of Jupiter discovered by Galileo, O. Römer was able to estimate the speed of light at 2.3 x 108 m/s. A more precise measurement was obtained using an experimental array devised by Léon Foucault: 2.98 x 108.
The speed of light in empty space and in air is generally rounded up to 3 x 108 m/s or 300,000 km/s. This means that light takes around 1.3 seconds to travel from the Moon to the Earth and about 81⁄3 minutes to reach the Earth from the Sun.
Light takes 4.3 years to reach our planet from the fixed star Alpha in Centaurus, about 2,500,000 years from the Andromeda nebula and more than 5 billion years from the most distant spiral nebulae.
Different theories of light enable us to describe observed regularities and effects. The corpuscular or particle theory of light, according to which units of energy (quanta) are propagated at the speed of light in a straight line from the light source, was proposed by Isaac Newton.
The wave theory of light, which suggests that light moves in a similar way to sound, was put forward by Christiaan Huygens. For more than a hundred years, scientists could not agree which theory was correct. Today, both concepts are used to explain the properties of light:
It was Newton again who discovered that white light contains colours. When a narrow beam of light is directed onto a glass prism and the emerging rays are projected onto a white surface, the coloured spectrum of light becomes visible.
In a further experiment, Newton directed the coloured rays onto a second prism, from which white light once again appeared. This was the proof that white sunlight is the sum of all the colours of the spectrum.
In 1822, Augustin Fresnel succeeded in determining the wavelength of light and showing that each spectral colour has a specific wavelength. His statement that “light brought to light creates darkness” sums up his realization that light rays of the same wavelength cancel each other out when brought together in corresponding phase positions.
E = h x ν
The energy E of an energy quantum (of radiation) is proportional to its frequency ν , multiplied by a constant h (Planck‘s quantum of action).
The Earth‘s atmosphere allows visible, ultra-violet and infrared radiation to pass through in such a way that organic life is possible. Wavelengths are measured in nanometres (nm) = 10-9 m = 10-7 cm. One nanometre is a ten-millionth of a centimetre.
Light is the relatively narrow band of electromagnetic radiation to which the eye is sensitive. The light spectrum extends from 380 nm (violet) to 780 nm (red). Each wavelength has a distinct colour appearance, and from short-wave violet through blue, green, green-yellow, orange up to long-wave red, the spectrum of sunlight exhibits a continuous sequence.
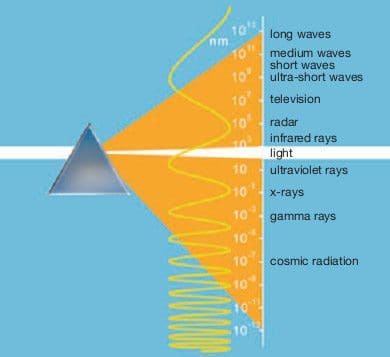
Coloured objects only appear coloured if their colours are present in the spectrum of the light source. This is the case, for example, with the sun, incandescent lamps and fluorescent lamps with very good colour rendering properties. Above and below the visible band of the radiation spectrum lie the infrared (IR) and ultraviolet (UV) ranges.
The IR range encompasses wavelengths from 780 nm to 1 nm and is not visible to the eye. Only where it encounters an object is the radiation absorbed and transformed into heat. Without this heat radiation from the sun, the Earth would be a frozen planet.
Today, thanks to solar technology, IR radiation has become important both technologically and ecologically as an alternative energy source.
For life on Earth, the right amount of radiation in the UV range is important. This radiation is classed according to its biological impact as follows:
- UV-A (315 to 380 nm), suntan, solaria;
- UV-B (280 to 315 nm), erythema (reddening of the skin), sunburn;
- UV-C (100 to 280 nm), cell destruction, bactericidal lamps.
Despite the positive effects of ultraviolet radiation – e.g. UV-B for vitamin D synthesis – too much can cause damage. The ozone layer of the atmosphere protects us from harmful UV radiation, particularly from UV-C.
If this layer becomes depleted (ozone gap), it can have negative consequences for life on Earth.










Dear Edvard,
After long time good article published from you on Lighting. I hope more articles on Electrical Lighting in future. Thank you very much for your Effort.
The last sentence in article ‘If this layer becomes depleted (ozone gap), it can have negative consequences for life on Earth.’ is very important nowdays regarding safety. As we all know ozone layer is pretty screwed up, so UV becomes very dangerous for humans on earth.